The material is actually a ‘dual-ion conductor’, which allows both protons and oxide ions to defuse, and can “realise high total conductivity at lower temperatures and improve the performance of electro-chemical devices”, according to TItech.
Dual-ion conducting materials such as Ba7Nb4MoO20 are known but “their conductivities are not high enough for practical applications”, said the Institute, adding: “And their underlying conducting mechanisms are not well understood.”
The research team decided to riff on that particular known material, adjusting the molybdenum fraction with compositions following the formula Ba7Nb4-xMo1+xO20+x/2.
One of those excelled both proton and oxide-ion conduction.
“Ba7Nb3.8Mo1.2O20.1 exhibited bulk conductivities of 11mS/cm at 537°C under wet air and 10mS/cm at 593°C under dry air,” said research head Professor Masatomo Yashima. “Total direct current conductivity at 400°C in wet air was 13 times higher than that of Ba7Nb4MoO20, and the bulk conductivity in dry air at 306°C is 175 times higher than conventional yttria-stabilized zirconia.”
To find out why it worked so well, TItech teamed up with the Australian Centre for Neutron Scattering, the University of Sydney, KEK (the Japanese High Energy Accelerator Research Organization), Tohoku University and the Japan Science and Technology Agency.
This formidable team embarked on molecular dynamics simulations, neutron diffraction analysis and neutron scattering length density analyses to discover an exotic game of pass-the-parcel.
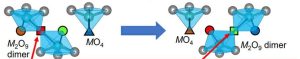
“The breaking and reforming of these dimers gives rise to ultrafast oxide-ion movement in a manner analogous to a long line of people relaying buckets of water from one person to the next,” said TItech. “Furthermore, simulations revealed that the observed high proton conduction was due to efficient proton migration in the hexagonal close-packed BaO3 layers in the material.”
“The present findings of high conductivities and unique ion migration mechanisms will help the development of science and engineering of oxide-ion, proton, and dual-ion conductors,” concluded Yashima.
The work is described n ‘Dimer-mediated cooperative mechanism of ultrafast-ion conduction in hexagonal perovskite-related oxides‘ in the journal Chemistry of Materials, all of which can be viewed without payment.
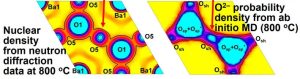
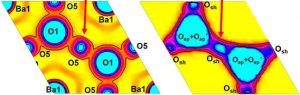
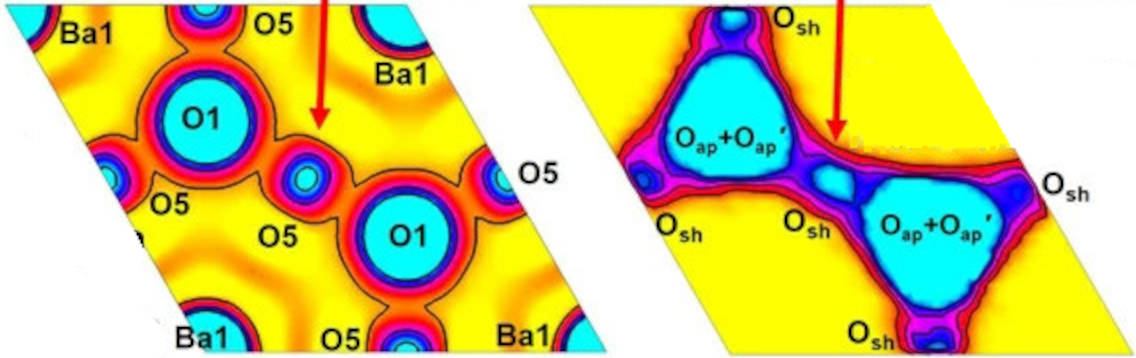
Red arrows: The interstitial ‘O5’ atom (left) corresponds to the corner-sharing oxygen atom ‘Osh’ (right) figure – and the red or green squares in the figure part way down the article).